INTRODUCTION
Arterial tourniquet use within the current Russo-Ukrainian conflict has resulted in alarmingly high rates of avoidable iatrogenic harm. Large-scale limb amputation and loss of life have resulted from the rigid application of Tactical Combat Casualty Care (TCCC) tourniquet principles within a mobility-contested battlespace, where forward tourniquet de-escalation capability has been deficient. Based on these observations, the ADF requires a robust and urgent rethink about how we manage arterial tourniquets in preparation for future combat operations.
Tourniquet de-escalation refers to a spectrum of procedural skills and knowledge tools used to minimise or eliminate the harm associated with tourniquet ischaemia. Tourniquet de-escalation may be organised into a three-tiered framework according to procedural complexity and provider capability (Figure 1). De-escalation Skills aim to directly restore vascular supply or facilitate reversal of vascular compromise. De-escalation Adjuncts are ancillary tools used for optimising tissue oxygenation and managing the systemic consequences of ischaemic reperfusion (Figure 2).
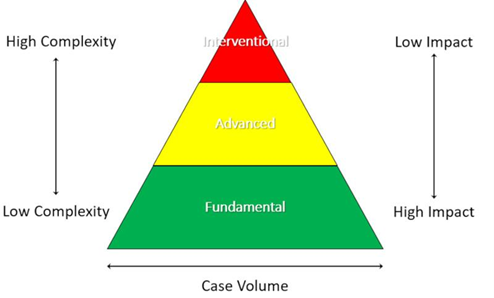
Figure 1: Organisational framework for tourniquet de-escalation (Green: Fundamental; Yellow: Advanced; Red: Interventional).
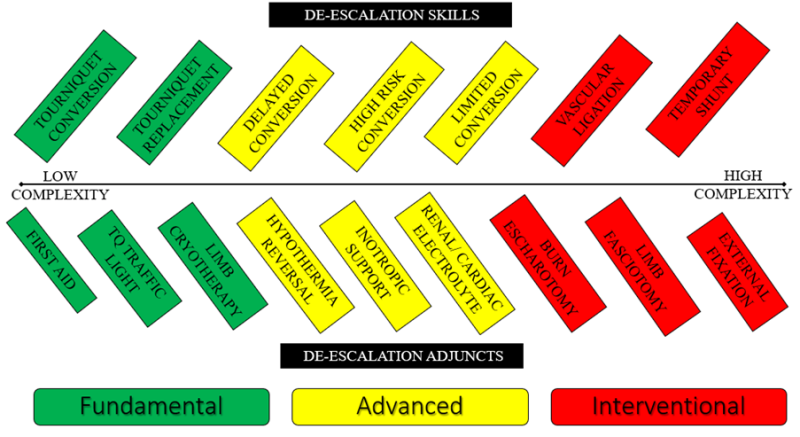
Figure 2: Tourniquet de-escalation skills and adjuncts according to complexity and organisational framework (Green: Fundamental; Yellow: Advanced; Red: Interventional).
Previous articles published within The Cove have developed a variety of concepts relating to tourniquet ischaemia and extended duration tourniquet application in prolonged field care. This article connects a number of these concepts to provide a comprehensive overview of Advanced Tourniquet De-escalation procedures. The training elements and knowledge tools outlined within this article are intended for use by advanced health care professionals, building upon the principles outlined within Fundamental Tourniquet De-escalation.
Advanced Tourniquet De-escalation may be organised into three phases: Assess, Convert, and Mitigate.
PHASE 1: ASSESS
Tourniquet de-escalation risk assessment is influenced by both time and casualty related factors.
The Tourniquet Traffic Light provides a framework for the common understanding of the time dependent ischaemic consequences associated with tourniquet application (Figure 3). In addition to predicting ischaemic injury, tourniquet application time also influences the risks of developing post-conversion haemodynamic instability and systemic reperfusion syndromes.
For the purposes of risk stratification after tourniquet conversion, Delayed Tourniquet Conversion is defined as release after 2 hours of application, reflecting the increased incidence of clinically meaningful vasomotor dysfunction and hypotensive events (Figure 3). High Risk Delayed Tourniquet Conversion is defined as tourniquet release after 4 hours of application, where life threatening metabolic effects may also be observed in addition to the risks of recurrent haemorrhage and vasomotor dysfunction (Figure 3, Figure 4). Tourniquet conversion beyond 6 hours duration of warm ischaemia is relatively contraindicated, with amputation above the level of tourniquet application recommended in most situations.
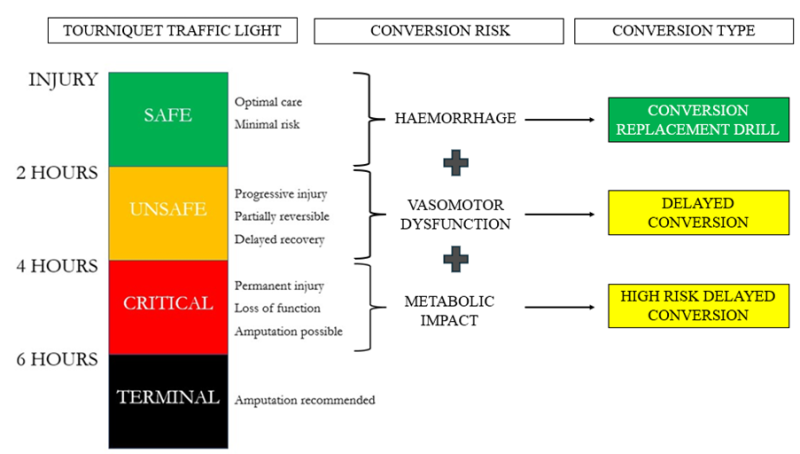
Figure 3: Tourniquet Traffic Light (left) relationship to post conversion pathophysiology (centre) and tourniquet conversion type (right). The Tourniquet Conversion-Replacement Drill (Green: Fundamental) is suitable for all-corps conduct within the first 2 hours of tourniquet application. Delayed Tourniquet Conversion and High Risk Delayed Tourniquet Conversion (Yellow: Advanced) require the capability to manage post-conversion hypotension and reperfusion syndromes.
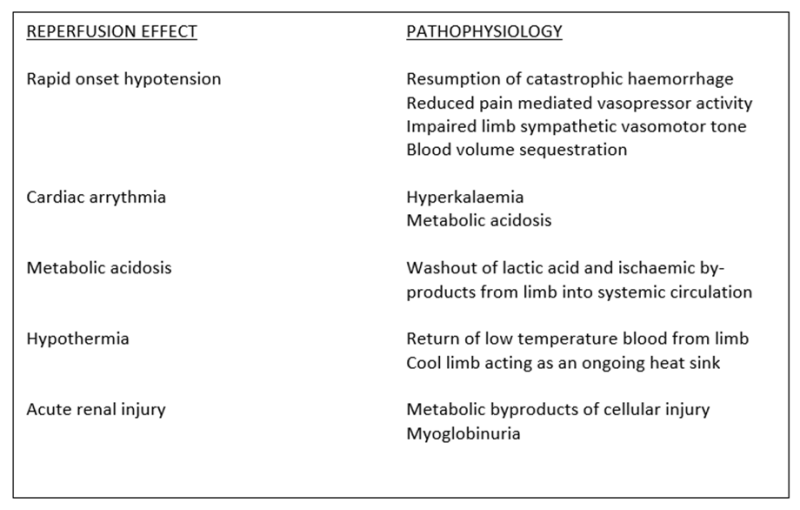
Figure 4: Tourniquet reperfusion pathophysiology. The metabolic risk profile is most significant during the conduct of High Risk Delayed Tourniquet Conversion (4-6 hours warm ischaemia time).
Evaluating a casualty for the suitability of tourniquet conversion after an extended duration of application requires a cautiously balanced approach between minimising ischaemia time and adequate resuscitation in anticipation of the haemodynamic and metabolic impacts of reperfusion. Advanced tourniquet conversion should therefore be viewed as a forced elective procedure, ideally conducted in a controlled environment after the institution of proactive resuscitative measures.
Management of higher priority injuries in a hemodynamically unstable casualty with polytrauma may necessitate delay in the conduct of tourniquet conversion. Balanced against the pursuit of an ideal resuscitation standard though is the progressive risk associated with accumulative time delay in the conduct of tourniquet conversion. Sound clinical judgement must be exercised as to when adequate (but not necessarily perfect) conditions have been obtained. Where conversion cannot be conducted within six hours of warm ischaemic time due to either clinical or tactical constraints, the tourniquet should remain in place, with view to performing primary amputation above the level of the tourniquet when circumstances allow.
PHASE 2: CONVERT
Techniques used to mitigate the combined haemodynamic and metabolic effects of ischaemic limb reperfusion are outlined within the Reperfusion Toolbox (Figure 5). Not all elements of the Reperfusion Toolbox will be required for every casualty. Resuscitation measures instituted prior to tourniquet conversion will be determined by individual casualty requirements and the duration of limb ischaemia.
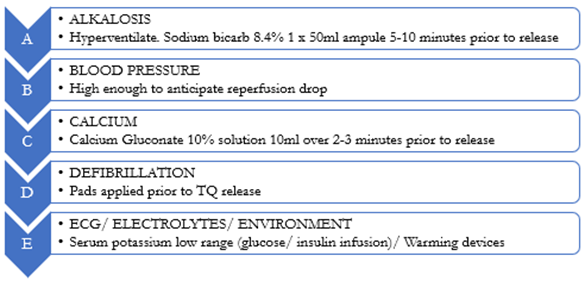
Figure 5: The Reperfusion Toolbox. Proactive resuscitation is required in preparation for tourniquet conversion after an extended duration of application.
Delayed Tourniquet Conversion (2-4 hours of warm ischaemia time)
Mild transient hypotension after tourniquet conversion is relatively common, even after relatively short durations of tourniquet application (beyond 30 minutes). A typical post-conversion hypotensive episode manifests as a reduction of systolic blood pressure by 10-30 mmHg for 1-2 minutes after tourniquet release, spontaneously correcting without intervention. Reperfusion hypotension is of greater concern however if the casualty is under-resuscitated prior to tourniquet conversion or if hypotensive resuscitation strategies are being employed.
Should inotropic support be necessary after tourniquet release to temporarily maintain target systolic blood pressure (≥80mmHg or ≥110mmHg with associated traumatic brain injury), then single dose bolus inotrope administration will often be sufficient (Metaraminol 0.25-0.5mg IV or Ephedrine 9-12mg IV). When preparing for Delayed Tourniquet Conversion, optimise fluid resuscitation prior to release and have inotropic support medication drawn ready for administration. Vasomotor disturbance observed after longer durations of tourniquet ischaemia may require ongoing inotropic support by infusion.
Medical countermeasures employed for tourniquet conversion within four hours of ischaemia are primarily directed towards preparatory resuscitation and the management of post-conversion hypotension. The systemic metabolic effects of ischaemic limb reperfusion are typically less significant with warm ischaemia times of less than four hours due to the time required to accumulate widespread cellular injury.
High Risk Delayed Tourniquet Conversion (4-6 hours of warm ischaemia time)
High Risk Delayed Conversion reflects the increased risks of metabolic reperfusion syndromes after tourniquet release. Metabolic reperfusion risk is determined by cellular injury and capillary dysfunction. Metabolic effects of limb reperfusion are therefore negligible at two hours of warm ischaemia (no cellular necrosis) but maximal at six hours (large scale cellular necrosis).
As muscle tissue is particularly prone to ischaemic injury, the metabolic impact of ischaemic reperfusion is typically more significant in the lower limb due to the larger volumes of muscle tissue involved. When preparing for High Risk Delayed Tourniquet Conversion, additional measures are necessary to mitigate the impacts of hyperkalaemia, acidosis, myoglobinuria, hypothermia, and cardiac dysfunction (Figure 4). All measures outlined within the Reperfusion Toolbox should therefore be considered prior to the conduct of High Risk Delayed Tourniquet Conversion (Figure 5).
Interventional Skills and Adjuncts to Advanced Tourniquet Conversion
Within appropriate clinical scope of practice an advanced health care provider may consider the conduct of Interventional De-Escalation measures to further improve limb perfusion and oxygenation if required.
Where tourniquet conversion has been successfully undertaken but circumferential full thickness or deep dermal burns continue to threaten distal limb perfusion, escharotomy should be conducted according to EMSB guidelines. Burn escharotomy is suitable for conduct in the pre-hospital care setting by non-surgeon providers.
The risk of post ischaemic compartment syndrome increases beyond three hours of tourniquet time. Compartment syndrome is largely a clinical diagnosis made by the observation of disproportionate pain, exacerbated by stretching of the involved musculature. A low threshold should be adopted for the diagnosis of compartment syndrome after a prolonged duration of arterial tourniquet application.
Definitive treatment of post ischaemic compartment syndrome is by early open fasciotomy of all myofascial compartments involved below the level of the tourniquet (Interventional De-Escalation; Figure 2). Where both escharotomy and fasciotomy are required to optimise tissue perfusion, fasciotomy incisions should be chosen to align with overlying escharotomy maps. There is no place for the expectant non-surgical treatment of compartment syndrome with potential myonecrosis after penetrating trauma as the ischaemic compartments have already been contaminated.
Local haemorrhage control by large vessel ligation may enable tourniquet release and thereby achieve distal limb perfusion by collateral arterial supply. Although not delivering direct restoration of vascular continuity in comparison to arterial repair or temporary shunting, large vessel ligation is relatively easy to perform and may allow enough distal perfusion to maintain limb viability in prolonged field care.
PHASE 3: MITIGATE
Where tourniquet conversion is unable to be performed, the iatrogenic harm associated with tourniquet ischaemia can still be reduced and outcomes improved by mitigation measures. In this regard, the principles of Fundamental Tourniquet De-Escalation should be applied. Tourniquet Replacement to the lowest appropriate level to preserve limb length and limb cryotherapy to reduce tissue metabolic rate will maximise the chances of cellular viability.
Limited Tourniquet Conversion may be considered after severe vascular injury where appropriate clinical, logistic, and tactical parameters are met. Limited Tourniquet Conversion involves the dynamic movement between tourniquet application states to achieve both haemorrhage control and enable distal limb perfusion (Figure 6). Even after major vascular injury, the combined effects of arterial vasospasm, permissive systemic hypotension, and compression wound packing may often be enough to significantly reduce or completely arrest haemorrhage flow rates.
Converting an arterial tourniquet in such situations, even if only for intermittent reperfusion intervals or by a partial reduction in tourniquet pressure, may maintain distal limb viability by collateral arterial supply. For intermittent tourniquet release, the first reperfusion interval is considered after two hours of tourniquet application. Reperfusion intervals of at least 10 minutes are required and are repeated every 90 minutes for the upper limb or repeated every 120 minutes for the lower limb.
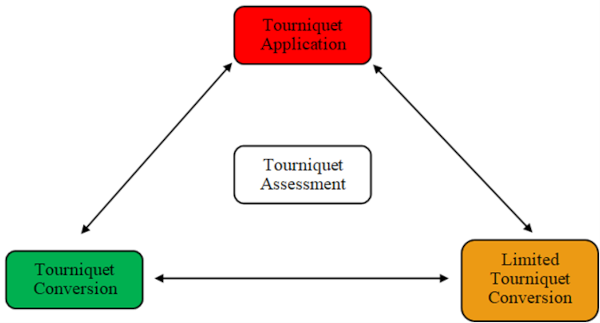
Figure 6: Tourniquet Application States. Limited Tourniquet Conversion by intermittent release or tourniquet pressure reduction should only be considered within suitable parameters of casualty resuscitation and resource capability.








Agreed. In a situation where collateral reperfusion can be facilitated by vessel ligation to enable tourniquet release, the capability of a treating facility needs to be weighed against the risks associated with ongoing limb ischemia. Where transfer to an advanced surgical care facility can not be achieved within suitable time frames (both in terms of local tissue damage and systemic reperfusion injury), then the conduct of interventional de-escalation procedures at a Role 1 facility certainly has justification. Delivery of advanced TQ de-escalation techniques forward in the battlespace however first requires implementation of a suitable training framework.